Description
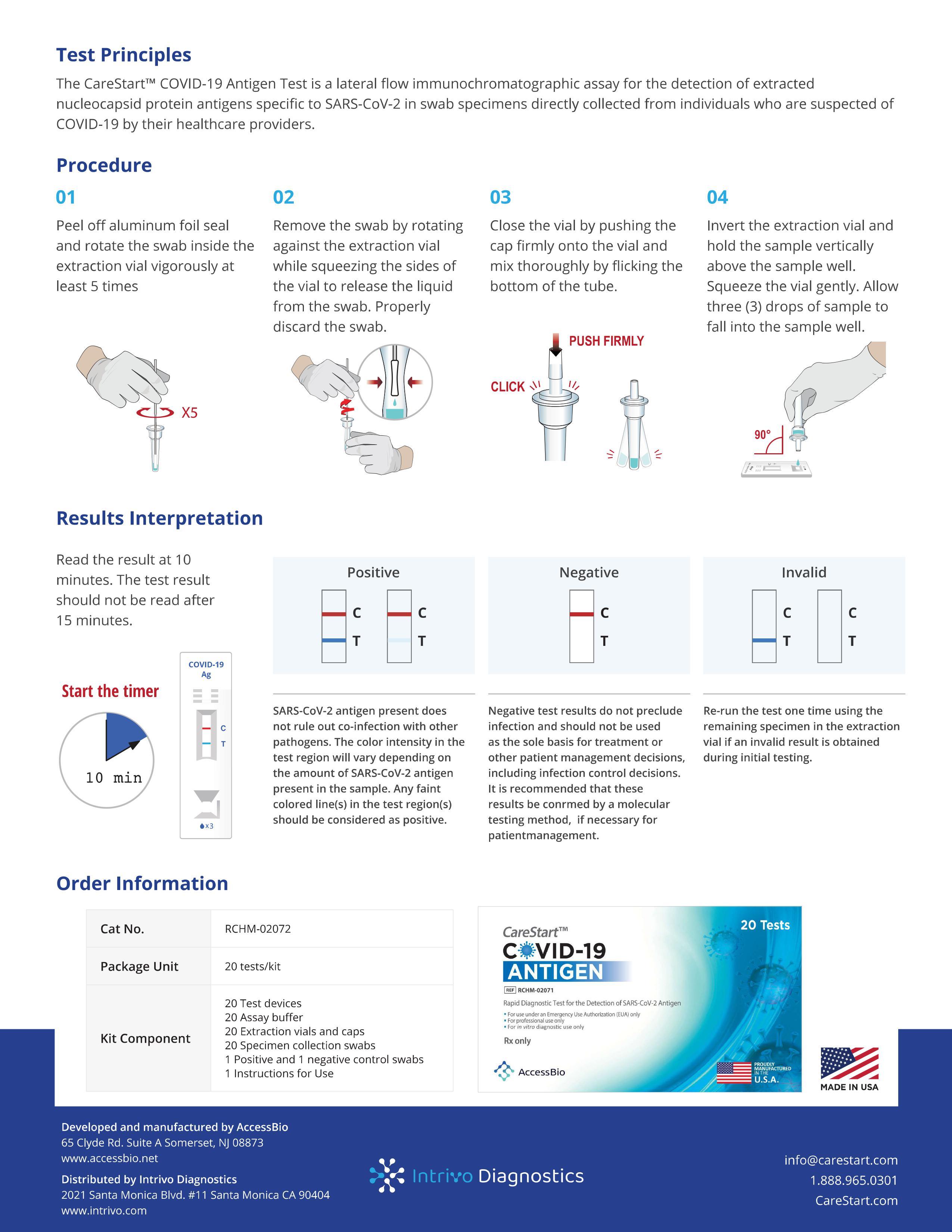
- Lateral flow assay
- Rapid results in 10 minutes
- Nasopharyngeal swab specimen collection
- Intended at POC setting (i.e., in patient care settings) by medical professionals operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation
- Detect SARS-CoV-2 nucleocapsid protein antigen
- Identify acute infection in symptomatic patients with 88.4% sensitivity and 100% specificity
- Designated as a CLIA waived tests
- Product can be fulfilled from New Jersey, Colorado or Florida